
Focused BBs Library
Targeted BBL- over 3000 unique PPIs, 3D Chemotypes and Anaplastic lymphoma kinase modulators
PPI Focused BBs Collection
The recently published research would suggest that PPI inhibitors are typically larger and more lipophilic than inhibitors of more standard binding sites of most proteins. One of the promising hypotheses is a combination of highly hydrophobic core within peripheral, more lipophilic, substituents. Taking into account overloaded chemical space of bioactive compounds, with sp2-rich aromatic structures which often cause toxicity and poor metabolic activity, we propose carefully designed and reasonably selected sets of hydrophobic and more three-dimensional BBs (sp3-enriched), that could be used as cores in synthesis of potential PPI inhibitors.
Over the years our team has been working on the design and optimization of synthetic procedures of novel Fsp3-enriched Building Blocks with multiple points of diversification, resulting in over 400 unique chemotypes. We have applied a combination of filtering parameters with molecular descriptors for evaluation of diversity and building blocks assessment as promising PPI drug-like cores. The following parameters were applied for the selection:
| PPI BB Library | Range | Average Value |
| Fsp3 | ≥ 0.7 | 0.87 |
| cLogP | < 3 | 1.2 |
| THSA (hydrophobic) | - | 368.5 Å2 |
| H-acceptors | ≤ 4 | 3.2 |
| H-donors | ≤ 3 | 1.2 |
| Diversity coefficient Td | ≤ 75 % | 70 % |
| No more than 1 Sulfur atom | ||
| no ureas, thioureas | ||
| common and abundant structures are removed | ||
PPI building blocks library.zip
3D BBs Collection
The vast majority of existing drugs has centered on sp2-rich aromatic cores. Moreover, often, we could find the same core fragment in several drugs with a different targeted diseases resulting in low specificity (selectivity) and rise of side effects. An expanded use of Building Blocks that possess more 3D character would provide access to a larger chemical space of bioactive compounds and drugs than those currently used. Nature recognizes small molecules in a complementary 3D fashion. The use of more complex and Fsp3-rich (more 3D) compounds would certainly increase existing chemical space, which might in turn be advantageous in exploring more demanding biological target. Although most of commercial libraries are diverse, selected to contain a good balance of properties, they all tend to have limited shape diversity. This ‘flatness’ could explain why they are less successful in identifying hits for certain targets that might require pharmacophores having alternative substitution vectors to interact with these proteins. Increasing diversity of building blocks in pharmaceutical chemistry aims to efficiently generate a set of molecules diverse skeletal, stereochemical and respectively in bioactivity properties.
We have carefully designed for your purposes the set of Novel and Diverse Building Blocks with highly sp3-rich skeletons. The following basic criteria were used to improve 3D shape functionality of our core building blocks:
Fsp3 > 0.65
at least 1 chiral center
TPSA < 80 Å2
checking for the sufficient novelty
easily functionalization (ideally 2 points of functionalization)
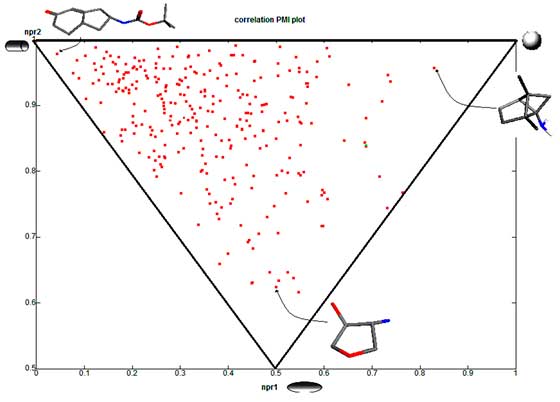
final selection based on distribution in PMI plot
Total number of building blocks in the library: 453
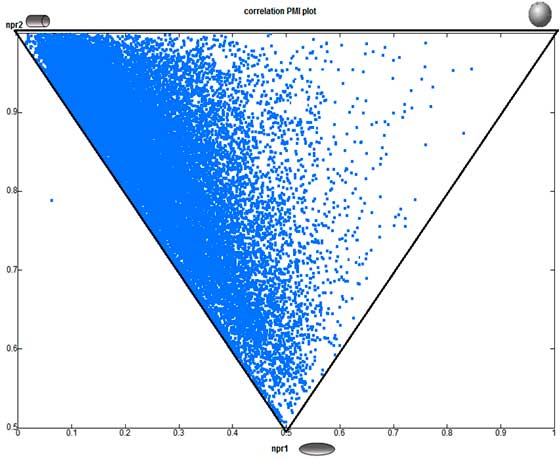
The PMI chart demonstrate that “3D Shape Space” coverage of Princeton BioMolecular’s 3D BBs dataset (shown as red dots) is more efficient and highly diverse in comparison to commercially available building block database of 56,974 compounds (blue points; Zbb Now, http://zinc.docking.org/subsets/zbb-now).
More information and examples can be found from the downloaded files:
New Products
-
Unique Organic Intermediates
Unique and popular organic BBL available in 24 hours shipping or synthesis on demand.
-
New Screening collection
Screening compounds from shelves in the client’s preferred format.
-
Tangible Arrays
Сarefully selected and designed based on biological and physicochemical properties of molecules.

